Data Analytics

Clinical Trial Systems

Full Clinical Services
RISE ABOVE with the ultimate flexibility
and responsiveness in clinical trials
Prevail InfoWorks, the Full Service Tech-Enabled CRO with The Single Interface™
Highest Quality Clinical Trials through Experts and Innovations
Prevail InfoWorks helps life sciences companies achieve better clinical trial outcomes through a full range of exceptional clinical trial services and cutting-edge technologies that improve every aspect of your trial.
Our patented means for improved processes, oversight, and data quality give every trial the best chance for success.
The Single Interface®
Ultimate single sign-on environment for collaboration and exploration.

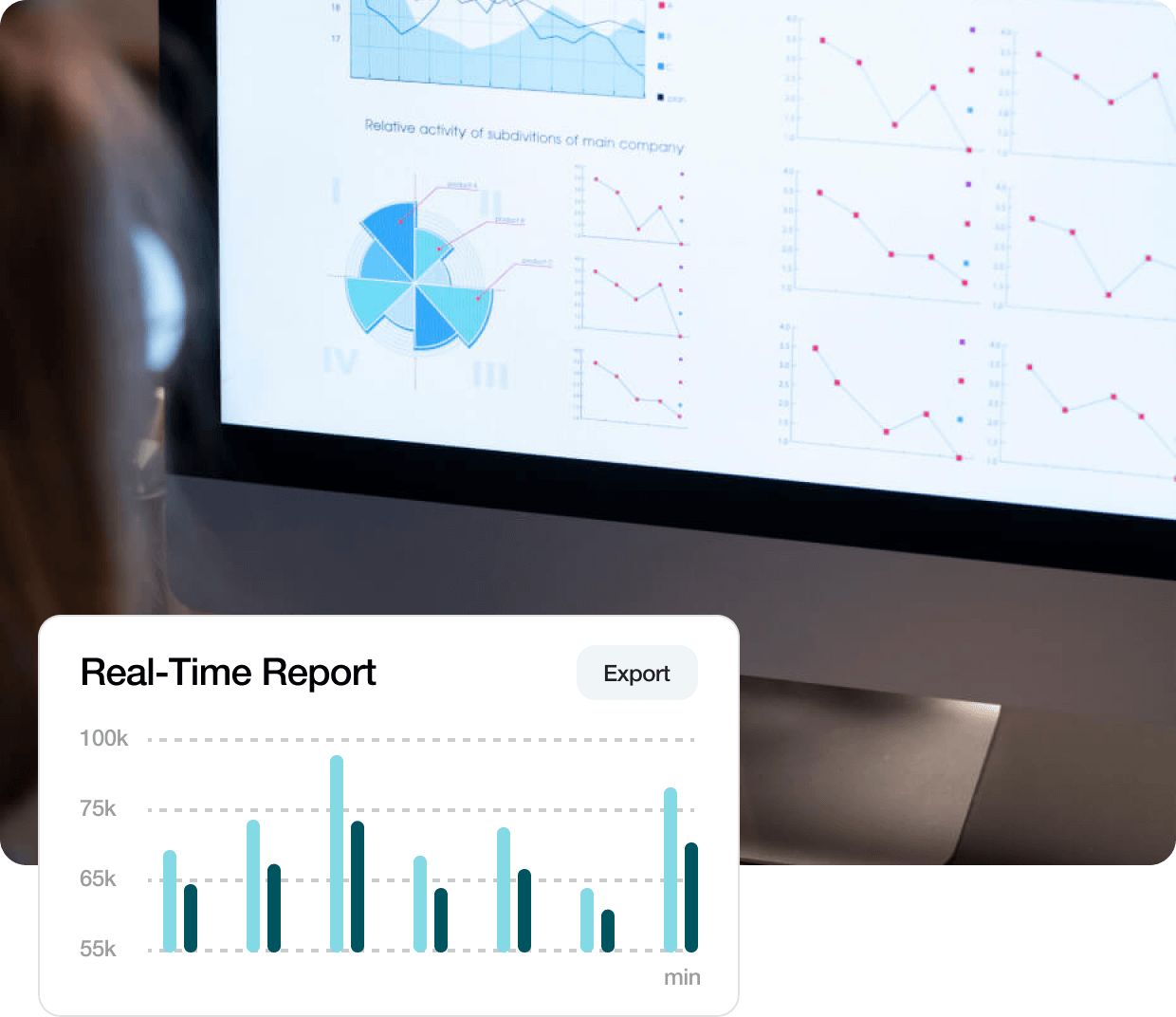
Integrated Analytics
Integrated Analytics
Improve study success with early observations from advanced analytics derived from the real-time integration of all your study and program data.
Electronic Data Capture & Clinical Data Management
Electronic Data Capture & Clinical Data Management
Get advanced, easy-to-use EDC for any budget.
Clinical Monitoring
Clinical Monitoring
Reduce operational risk with automated study progress tracking and early warnings.
Safety Data Management System with Early Safety Signal Detection
Safety Data Management System with Early Safety Signal Detection
Speed up the detection, analysis, reporting and reconciliation of safety events for your study or program.
eTMF & Document Management
eTMF & Document Management
Always be inspection-ready with an integrated system for the collection and filing of essential documents for clinical trial compliance and control.
IRT & RTSM
IRT & RTSM
Fast, flexible, and controlled randomization and trial supplies management while keeping your budget in check.
EPRO/eCOA
EPRO/eCOA
Make collection of patient-reported data easier to set up, track, manage, and ensure compliance.

Grant Navigator for Site Payments
Grant Navigator for Site Payments
Automate investigator grant invoicing, reconciliation, payments, and reporting to financially manage and control your study costs.
Clinical Adjudication
Clinical Adjudication
Improve accuracy and consistency of clinical endpoint adjudication data and streamline CEC review.
Clinical Trial Services by Experienced Professionals
Focused on the common goal of success in your clinical trial, our teams of exceptional multidisciplinary professionals can cover all of your service needs, from site identification to post-database lock statistical programming, or just specific functional areas in which you may need support.
Leveraging the power of The Single Interface®, Prevail’s clinical operation teams are uniquely advantaged with improved processes, real-time information, and the ability to manage complex issues for better outcomes.
Project Management
Contracting & Project Accounting
Site Management
TMF Management
Clinical Monitoring
Information & Data Management
Medical Monitoring
Pharmacovigilance
Clinical Supplies Management
What our customers say about us
Ready to achieve better
clinical outcomes?
Ready to see how Prevail’s services, clinical trial systems, and integrated analytics can help you improve clinical development and achieve better outcomes?
