CTMS & Clinical Monitoring
Reduce operational risk with automated study
progress tracking and early warnings

Reduce operational risk with automated study
progress tracking and early warnings
Your study needs automated workflow tools that reduce complexity and workload rather than increase it. Prevail’s integrated CTMS with Clinical Monitoring lets you easily track the progress of your study, including startup, enrollment, status of site data entry / monitoring / cleaning, upcoming patient visit dates, actual versus projected enrollment, and much more. Aggregated, cross-study visualizations let you easily identify critical trends and risks across a program instead of a single study.
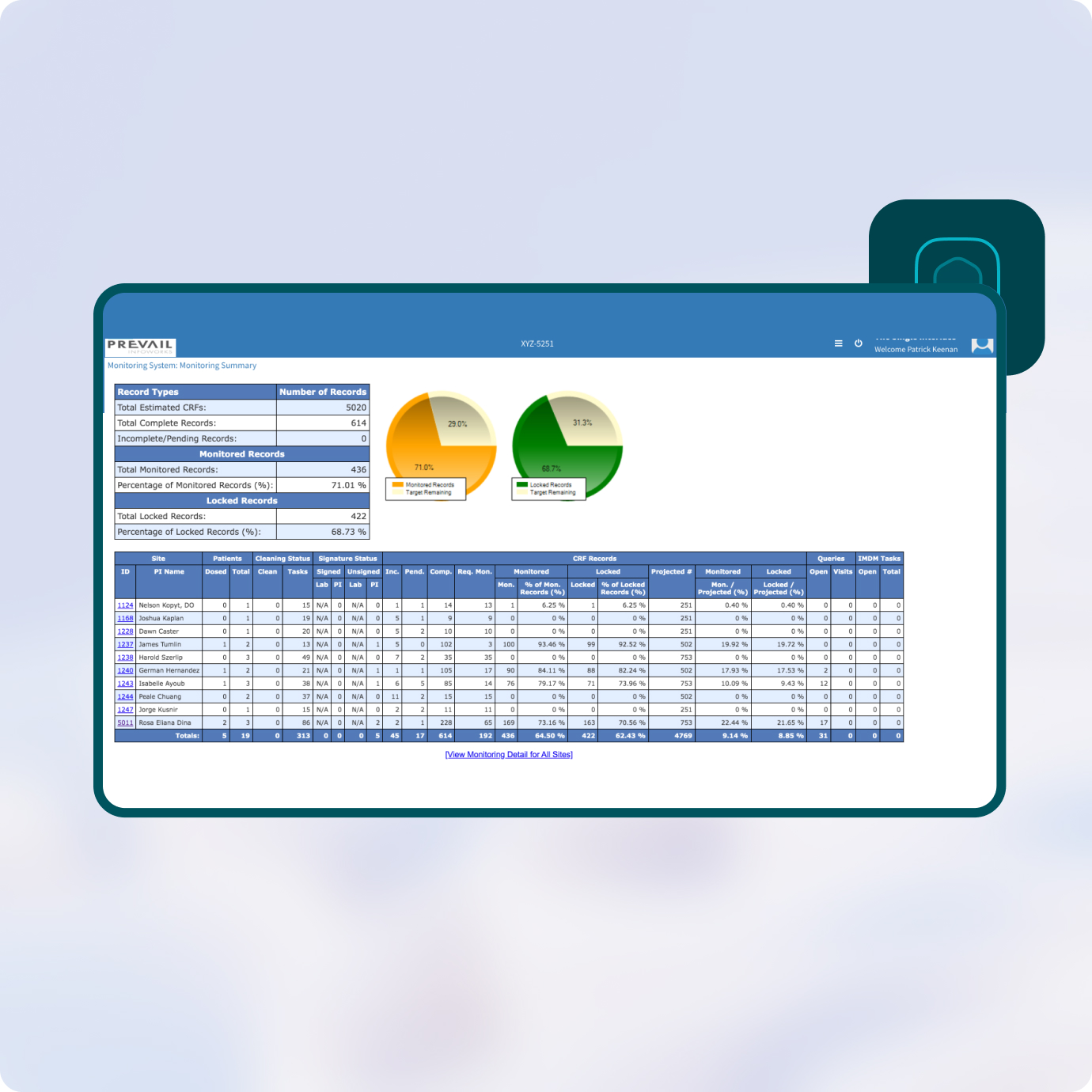
Prevail’s Clinical Monitoring provides advanced visibility to sponsors and project managers on overall work performed versus work remaining.
Your teams get real-time dashboards showing key monitoring metrics on recruitment, enrollment, SDV, CRF locking, query tracking and other indicators while allowing anyone to drill down to the site, patient, CRA or task level.
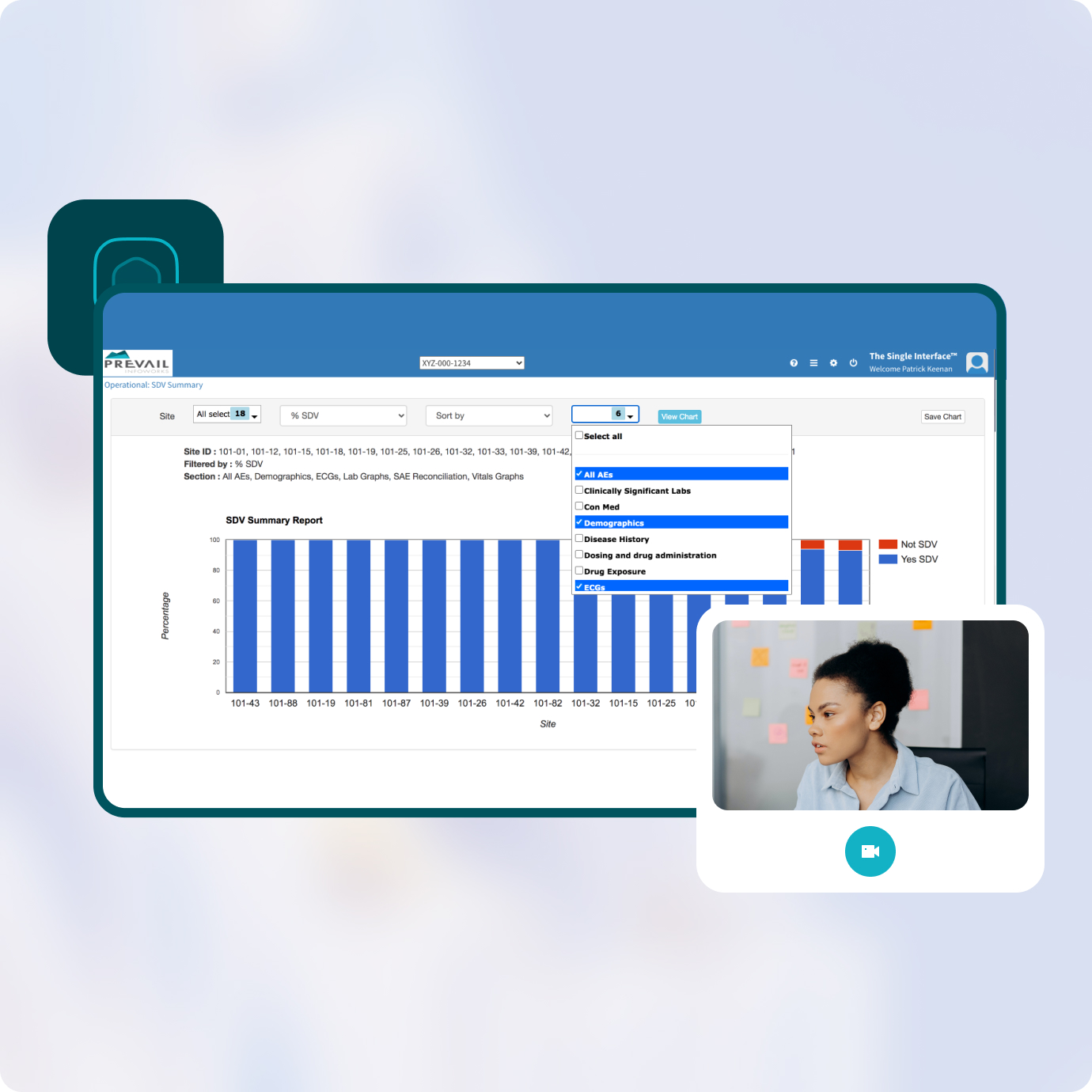
Dashboards provide each stakeholder with role-based information they need to oversee, assess, and perform clinical monitoring.
Instead of devoting precious time on-site to both identifying and solving problems, equip monitors with auto-generate task lists, a.k.a. “Don’t fly home without it!” lists.
Track monitoring reports by having CRAs upload documents directly into the system, allowing for remote review and approval.
Find room for improvement by viewing progress by site, monitor or patient. Notice an issue and drill-down to identify actionable details and assign tasks.
Learn how Prevail’s Clinical Monitoring can help make your study a success.

Ready to see how Prevail’s Clinical Monitoring
can reduce study risk, cost, and effort?