
Safety Data Management System
Speed up the detection, analysis, reporting and reconciliation
of safety events for your study or program

Speed up the detection, analysis, reporting and reconciliation
of safety events for your study or program
Prevail’s Safety System provides a validated safety database that automatically reconciles safety events with the clinical database.
Track adverse events from any place in the world, reduce manual effort and risk with fields that auto-populate straight from the clinical database, analyze safety in context across multiple study visits, and view concomitant medications’ impact on lab results or AEs without having to leave a single page.
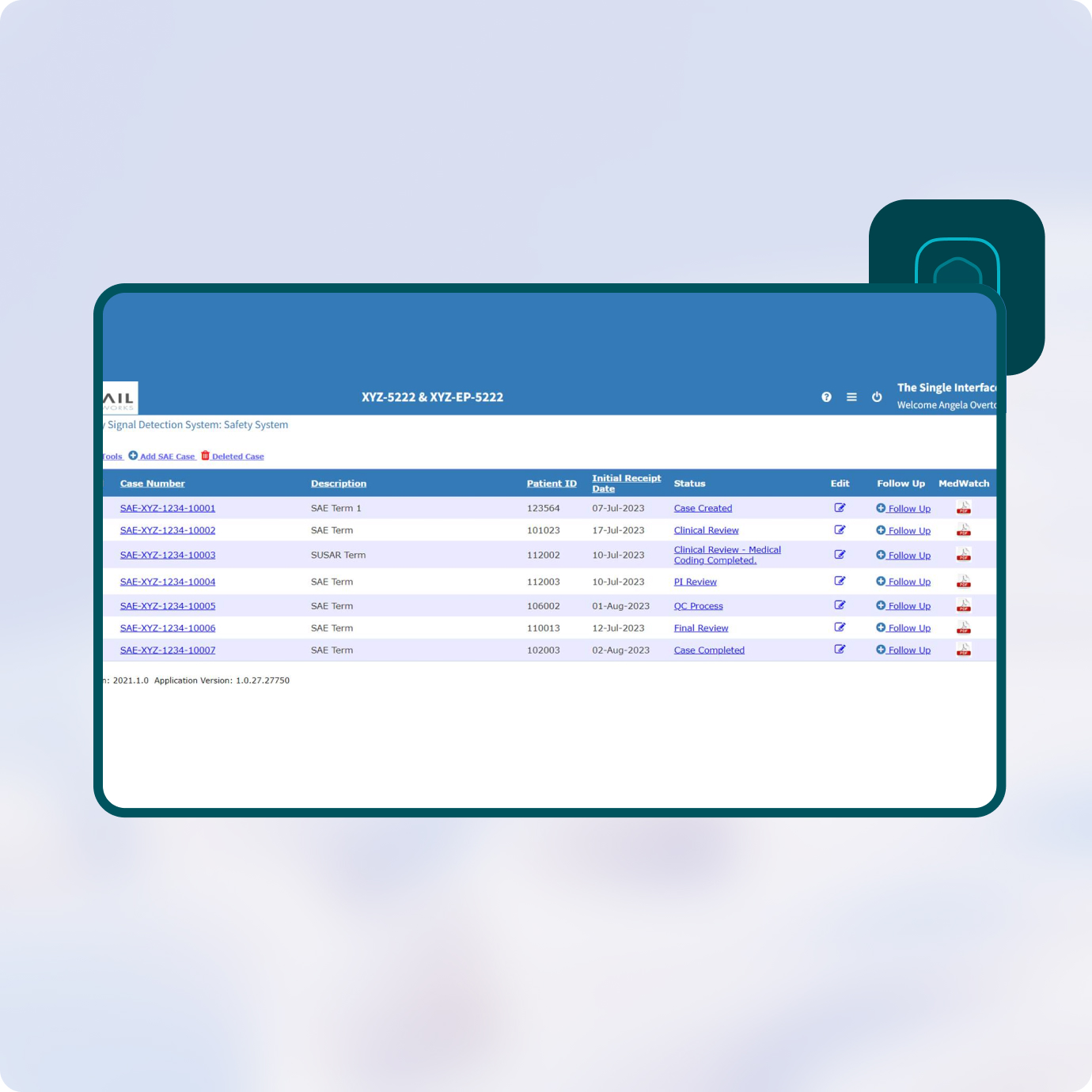
Prevail’s Safety System is a user-friendly, robust and powerful tool for tracking, managing and reporting Adverse Events and Serious Adverse Events.
Adding a new SAE Case to the safety database is simple – just click the “Add SAE Case” tab. The system then walks users through the necessary information that needs to be reported, from Subject Demographics to Medical Coding.
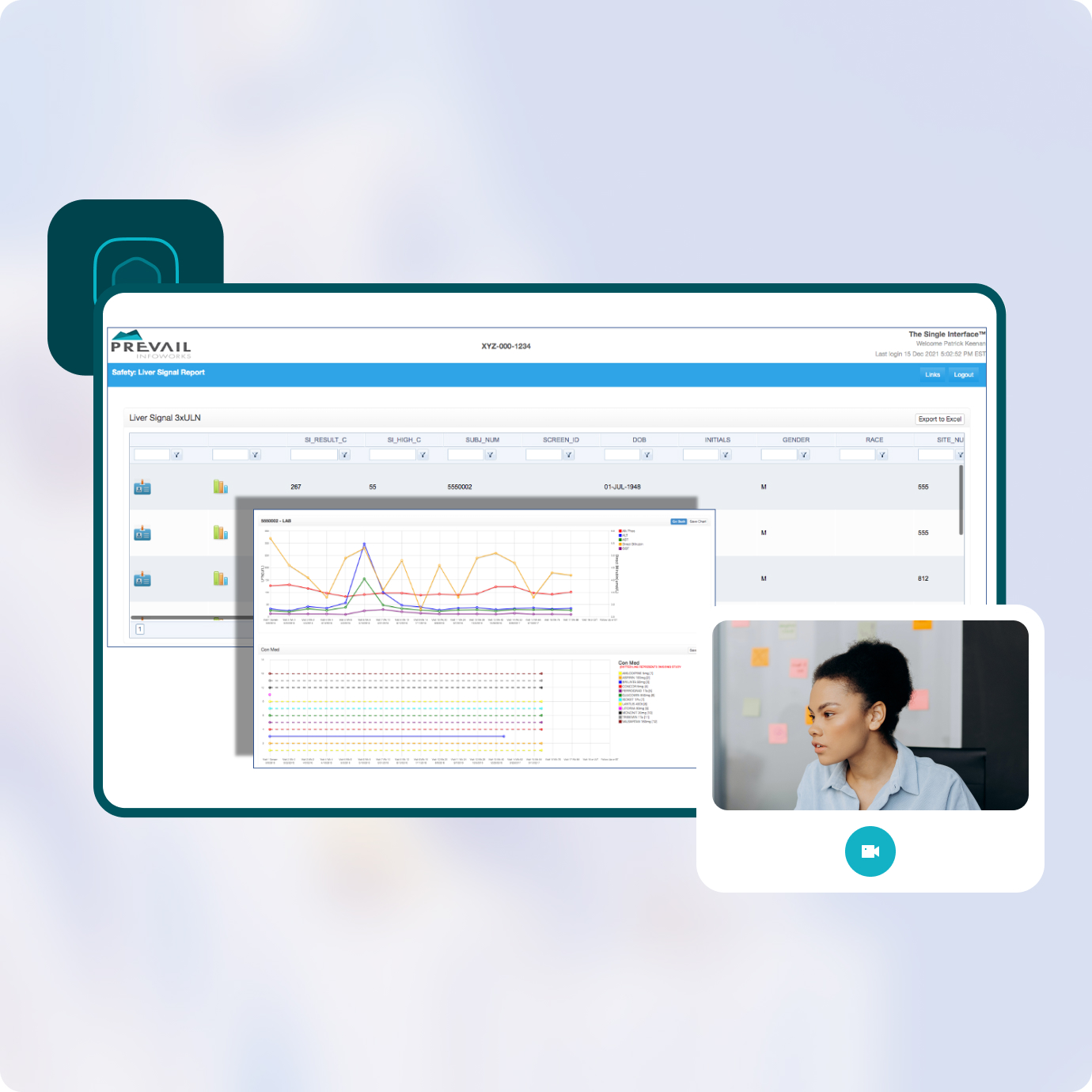
Typically a labor-intensive process of reconciling and correcting mismatches between two separate databases, the built in Safety Management System minimizes this task by auto-populating fields and providing built-in mismatch reports, thus eliminating duplication of entries and reducing time spent on reconciliation.
The Safety Data Management System automatically populates the MedWatch or CIOMS forms, reducing time spent on manual completion and eliminating the risk of human error.
Upon receipt of a safety event the platform enables instantaneous alerts to all required users without requiring human action. Complex analytics provide context to safety events and enable fast interpretation of safety labs, ECGs, diagnostics, concomitant medications and other CRF data.
Save costs across several studies and prevent expensive reconciliation of multiple databases by having a safety database that covers an entire product or program.
Learn how Prevail’s Safety Data Management System can help make your study a success.
Ready to see how Prevail’s Safety Management System can help improve patient safety and reporting compliance with less effort?